The evolution of orthopedic implants has reached unprecedented heights, with modern bone plate technology representing one of the most significant advances in surgical intervention. As medical professionals demand higher precision and better patient outcomes, manufacturers are leveraging cutting-edge techniques to create implants that exceed traditional performance standards. These sophisticated devices play a crucial role in fracture fixation, spinal fusion, and reconstructive procedures across diverse patient populations.
Manufacturing excellence directly impacts the clinical success of orthopedic procedures. Advanced production methodologies enable the creation of implants with superior mechanical properties, enhanced biocompatibility, and precise dimensional accuracy. These improvements translate to reduced surgical complications, faster healing times, and improved long-term patient satisfaction across various orthopedic applications.
Precision Engineering in Medical Device Manufacturing
Computer-Controlled Machining Systems
Modern manufacturing facilities utilize state-of-the-art computer numerical control systems to achieve micron-level precision in bone plate production. These sophisticated machines operate with tolerances that far exceed traditional manufacturing capabilities, ensuring each implant meets exact specifications. The integration of real-time monitoring systems allows for immediate quality adjustments during the production process.
Multi-axis machining centers enable the creation of complex geometries that were previously impossible to achieve through conventional methods. This technological advancement allows manufacturers to optimize plate designs for specific anatomical requirements while maintaining structural integrity. The precision achieved through these systems directly correlates with improved surgical outcomes and reduced revision rates.
Advanced Material Processing Techniques
Titanium alloy processing has evolved significantly with the introduction of specialized heat treatment protocols and surface modification techniques. These processes enhance the mechanical properties of the base material while improving osseointegration capabilities. Controlled atmosphere processing prevents contamination and ensures consistent material properties throughout each production batch.
Surface treatment innovations, including plasma spraying and electrochemical processes, create optimal surface textures for bone growth. These treatments improve the initial stability of the implant while promoting long-term biological fixation. The combination of advanced materials and sophisticated processing techniques results in implants that demonstrate superior performance across diverse clinical applications.
Quality Control and Testing Protocols
Non-Destructive Testing Methods
Comprehensive quality assurance programs incorporate multiple non-destructive testing methodologies to verify implant integrity without compromising the devices themselves. Ultrasonic testing reveals internal defects that could compromise performance, while radiographic inspection ensures dimensional accuracy. These testing protocols identify potential issues before products reach the surgical environment.
Advanced imaging techniques, including micro-computed tomography, provide detailed three-dimensional analysis of internal structures. This technology enables manufacturers to verify porosity levels, detect minute cracks, and confirm proper material distribution throughout each device. The implementation of these testing methods significantly reduces the risk of implant failure in clinical applications.
Mechanical Property Validation
Rigorous mechanical testing protocols simulate the extreme conditions that implants encounter within the human body. Fatigue testing machines subject devices to millions of loading cycles, replicating years of physiological stress in controlled laboratory environments. These tests validate the long-term durability of each bone plate design under realistic operating conditions.
Biomechanical analysis extends beyond basic strength testing to evaluate how implants interact with surrounding bone tissue. Advanced simulation software models the stress distribution patterns that occur during normal physiological activities. This comprehensive approach ensures that manufactured devices will perform optimally throughout their intended service life while minimizing the risk of mechanical complications.
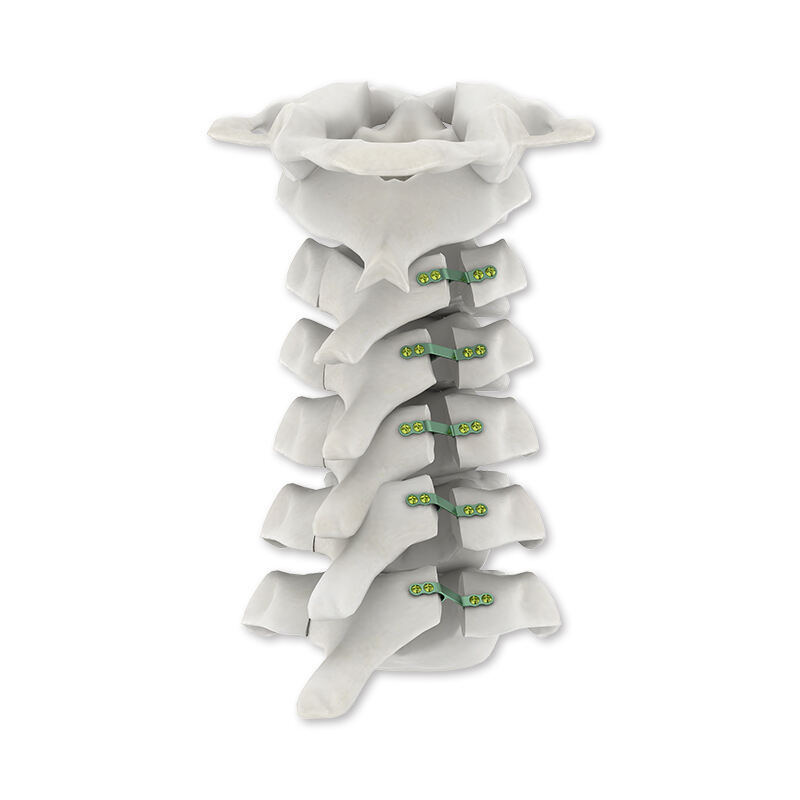
Innovation in Design and Development
Anatomically Optimized Configurations
Contemporary design methodologies incorporate extensive anatomical databases to create implants that match natural bone contours with exceptional precision. Three-dimensional modeling software enables engineers to optimize plate configurations for specific skeletal regions while maintaining biomechanical effectiveness. This patient-specific approach reduces surgical time and improves overall treatment outcomes.
Finite element analysis allows designers to predict how different configurations will behave under various loading conditions. This computational approach enables the optimization of hole patterns, plate thickness, and overall geometry before physical prototypes are manufactured. The result is a new generation of implants that demonstrate superior performance characteristics across diverse patient populations.
Modular System Development
Advanced manufacturing capabilities have enabled the development of comprehensive modular systems that provide surgeons with unprecedented flexibility during procedures. These systems incorporate standardized interfaces while offering numerous configuration options to address specific clinical requirements. The modular approach reduces inventory complexity while ensuring optimal solutions for diverse surgical scenarios.
Interchangeable components within these systems undergo rigorous compatibility testing to ensure reliable performance across all possible configurations. Manufacturing precision is critical to maintaining proper fit and function between different system elements. This systematic approach to product development results in comprehensive solutions that address the full spectrum of orthopedic reconstruction needs.
Regulatory Compliance and Standards
International Quality Standards
Manufacturing facilities must comply with stringent international standards that govern medical device production, including ISO 13485 certification and FDA Quality System Regulation requirements. These standards mandate comprehensive documentation of all manufacturing processes, from raw material receipt through final product distribution. Compliance ensures consistent quality and traceability throughout the production lifecycle.
Regular audits by regulatory bodies verify adherence to established protocols and identify opportunities for continuous improvement. These assessments evaluate manufacturing practices, quality control procedures, and documentation systems to ensure ongoing compliance. The rigorous oversight required for medical device manufacturing drives continuous innovation in production methodologies and quality assurance practices.
Biocompatibility Verification
Comprehensive biocompatibility testing protocols evaluate how manufactured devices interact with biological systems over extended periods. These studies assess cytotoxicity, sensitization potential, and long-term tissue response to ensure patient safety. Advanced testing methodologies provide detailed information about material performance in physiological environments.
Long-term clinical studies track the performance of manufactured implants across diverse patient populations and surgical applications. This data collection process provides valuable feedback for continuous improvement of manufacturing processes and design optimization. The systematic evaluation of clinical outcomes drives the development of increasingly effective orthopedic solutions.
Future Trends in Manufacturing Technology
Additive Manufacturing Integration
Three-dimensional printing technologies are revolutionizing the production of custom orthopedic implants, enabling patient-specific solutions that were previously impossible to manufacture economically. These advanced systems can create complex internal structures that optimize mechanical properties while reducing overall implant weight. The integration of additive manufacturing with traditional production methods expands design possibilities significantly.
Selective laser melting and electron beam melting processes enable the direct manufacture of titanium components with properties that match or exceed those of conventionally manufactured devices. These technologies reduce waste material while enabling the creation of optimized internal architectures. The continued development of additive manufacturing promises to transform orthopedic device production in the coming decades.
Smart Manufacturing Systems
Artificial intelligence and machine learning technologies are being integrated into manufacturing systems to optimize production parameters in real-time. These smart systems can predict equipment maintenance needs, adjust processing parameters for optimal quality, and identify potential defects before they occur. The implementation of Industry 4.0 concepts enhances both efficiency and quality in medical device manufacturing.
Internet of Things connectivity enables comprehensive monitoring of manufacturing environments, tracking parameters such as temperature, humidity, and contamination levels. This data collection provides valuable insights for process optimization and quality improvement initiatives. The evolution toward smart manufacturing systems represents the future of high-precision medical device production.
FAQ
What materials are commonly used in modern bone plate manufacturing
Modern bone plates are primarily manufactured from titanium alloys, particularly Ti-6Al-4V, due to their excellent biocompatibility, corrosion resistance, and favorable mechanical properties. Stainless steel grades such as 316L are also used in specific applications, while newer materials like tantalum and PEEK composites are being explored for specialized requirements. The material selection depends on the specific clinical application, required mechanical properties, and patient considerations.
How long does the manufacturing process typically take for orthopedic implants
The complete manufacturing cycle for orthopedic implants typically ranges from several weeks to several months, depending on complexity and testing requirements. Initial machining and forming operations may take days, while surface treatments, sterilization, and comprehensive quality testing extend the timeline significantly. Custom or patient-specific implants generally require additional time for design verification and specialized manufacturing setup.
What quality certifications are required for bone plate manufacturers
Bone plate manufacturers must obtain ISO 13485 certification for medical device quality management systems, along with regulatory approvals from relevant authorities such as FDA 510(k) clearance in the United States or CE marking in Europe. Additional certifications may include ISO 14971 for risk management and ISO 10993 for biological evaluation. These certifications require extensive documentation and regular audits to maintain compliance.
How do manufacturers ensure the sterility of bone plates
Manufacturers employ validated sterilization methods such as gamma irradiation, electron beam sterilization, or ethylene oxide gas treatment, depending on material compatibility and packaging requirements. Sterile packaging systems maintain sterility throughout distribution and storage. Comprehensive validation studies demonstrate the effectiveness of sterilization processes, and ongoing monitoring ensures consistent sterility assurance levels throughout production.